
Endometrial cancer, the most common gynecological malignancy in developed countries, demands effective prognostic tools to guide treatment decisions and improve patient outcomes. Prognostic biomarkers play a crucial role in predicting disease progression, recurrence, and patient survival, enabling personalized treatment strategies. This article explores the current and emerging prognostic biomarkers in endometrial cancer, highlighting their clinical relevance and potential to transform patient care.
Current Prognostic Biomarkers
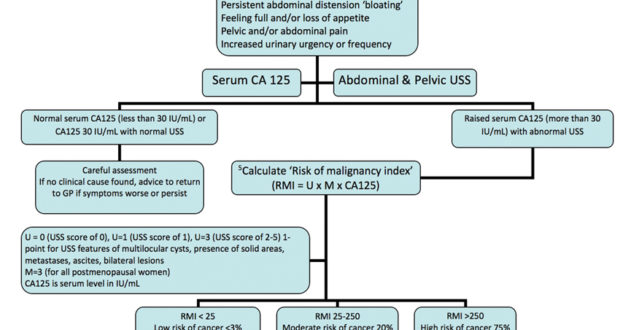
CA125 is a well-established biomarker used primarily in ovarian cancer but also relevant in endometrial cancer. Elevated levels of CA125 are associated with advanced-stage disease and poor prognosis. It is often used in combination with other biomarkers to improve prognostic accuracy.
- HE4 (Human Epididymis Protein 4)
HE4 has gained prominence as a biomarker in endometrial cancer due to its high specificity and sensitivity. Elevated HE4 levels correlate with aggressive tumor behavior, higher tumor grade, and advanced disease stage. HE4 is valuable in predicting disease recurrence and monitoring treatment response.
- p53
p53, a tumor suppressor gene, is commonly mutated in endometrial cancer. The presence of p53 mutations is associated with high-grade, high-risk endometrial carcinomas, and poor prognosis. Immunohistochemical staining for p53 helps identify patients with aggressive disease who may benefit from more intensive treatment.
- Ki-67
Ki-67 is a marker of cell proliferation. High Ki-67 expression is linked to increased tumor aggressiveness and poorer outcomes in endometrial cancer. It serves as a prognostic indicator, helping to stratify patients based on their risk of recurrence and survival.
- L1CAM (L1 Cell Adhesion Molecule)
L1CAM is associated with tumor invasion and metastasis. High L1CAM expression in endometrial cancer is linked to poor prognosis, increased risk of recurrence, and reduced survival rates. It serves as a valuable marker for identifying high-risk patients who may require more aggressive treatment.
Emerging Prognostic Biomarkers
- MSI (Microsatellite Instability)
Microsatellite instability, a result of defects in the DNA mismatch repair system, is emerging as an important prognostic marker in endometrial cancer. MSI-high tumors are associated with better overall survival and may respond well to immunotherapy. MSI testing can help identify patients who might benefit from immune checkpoint inhibitors.
- ARID1A (AT-Rich Interaction Domain 1A)
ARID1A is a tumor suppressor gene frequently mutated in endometrial cancer. Loss of ARID1A expression is associated with poorer prognosis and higher risk of recurrence. ARID1A status can provide valuable prognostic information and guide treatment decisions.
- PTEN (Phosphatase and Tensin Homolog)
PTEN is another tumor suppressor gene commonly mutated in endometrial cancer. Loss of PTEN function is linked to aggressive tumor behavior and poor prognosis. Assessing PTEN status can help predict disease outcomes and identify patients at higher risk of recurrence.
- CTNNB1 (Catenin Beta 1)
Mutations in the CTNNB1 gene, which encodes the beta-catenin protein, are associated with endometrial cancer. These mutations often indicate a more favorable prognosis, with lower risk of recurrence and better overall survival. CTNNB1 status can aid in risk stratification and treatment planning.
- miRNAs (MicroRNAs)
MicroRNAs are small non-coding RNAs that regulate gene expression and play a role in cancer progression. Specific miRNAs, such as miR-200c and miR-205, have been identified as potential prognostic markers in endometrial cancer. Altered expression of these miRNAs is associated with tumor aggressiveness and patient outcomes.
Integrating Biomarkers into Clinical Practice
The integration of prognostic biomarkers into clinical practice requires standardized testing protocols and robust validation studies. Multimodal approaches that combine multiple biomarkers can provide a more comprehensive risk assessment, improving the accuracy of prognostic predictions. Additionally, incorporating biomarkers into clinical decision-making tools can help tailor treatment strategies to individual patients, optimizing outcomes.
Future Directions
The future of prognostic biomarkers in endometrial cancer lies in the development of personalized medicine approaches. Advances in genomics, proteomics, and bioinformatics will facilitate the discovery of novel biomarkers and the refinement of existing ones. Liquid biopsies, which analyze circulating tumor DNA and other cancer-related molecules in blood, hold promise for non-invasive monitoring of disease progression and treatment response.
Moreover, the integration of artificial intelligence and machine learning into biomarker research can enhance predictive modeling, enabling more precise prognostication and treatment planning. Collaborative efforts between researchers, clinicians, and industry stakeholders will be crucial in translating these advancements into clinical practice.
Prognostic biomarkers are essential tools in the management of endometrial cancer, providing critical information on disease progression, recurrence risk, and patient survival. Current biomarkers like CA125, HE4, p53, Ki-67, and L1CAM, along with emerging markers such as MSI, ARID1A, PTEN, CTNNB1, and miRNAs, offer valuable insights into tumor biology and patient outcomes.
Continued research and technological advancements will further refine these biomarkers, enhancing their clinical utility and paving the way for personalized treatment strategies. By integrating biomarkers into routine clinical practice, healthcare providers can improve prognostication, tailor treatments, and ultimately, enhance the care and outcomes of patients with endometrial cancer.
Challenges and Considerations
While prognostic biomarkers in endometrial cancer hold immense promise, several challenges and considerations must be addressed:
- Standardization: Establishing standardized protocols for biomarker testing and interpretation to ensure consistency and reliability across different laboratories and healthcare settings.
- Validation: Conducting rigorous validation studies to confirm the clinical utility and predictive value of emerging biomarkers before integrating them into routine clinical practice.
- Cost-Effectiveness: Evaluating the cost-effectiveness of biomarker testing in relation to its impact on patient outcomes and healthcare resource utilization.
- Interdisciplinary Collaboration: Fostering collaboration among oncologists, pathologists, geneticists, and bioinformaticians to maximize the potential of biomarkers in guiding treatment decisions.
- Ethical Considerations: Addressing ethical issues related to biomarker testing, including patient consent, privacy concerns, and equitable access to testing and treatment options.
Future Directions in Biomarker Research
- Integration of Multi-Omics Approaches: Combining genomics, proteomics, metabolomics, and epigenomics to uncover comprehensive biomarker signatures that capture the heterogeneity of endometrial cancer and improve prognostic accuracy.
- Digital Health Technologies: Harnessing digital health tools for real-time monitoring of biomarkers and disease progression, enabling proactive management and personalized interventions.
- Machine Learning and AI: Applying artificial intelligence and machine learning algorithms to analyze complex biomarker data sets and develop predictive models for patient outcomes and treatment response.
- Liquid Biopsies: Advancing liquid biopsy techniques for non-invasive detection of circulating tumor biomarkers, facilitating early diagnosis, monitoring treatment response, and detecting minimal residual disease.
- Clinical Trials and Translational Research: Conducting prospective clinical trials to validate novel biomarkers and evaluate their impact on clinical decision-making and patient outcomes.
Prognostic biomarkers represent a cornerstone in the era of personalized medicine for endometrial cancer. They provide invaluable insights into tumor biology, guide treatment decisions, and enhance patient stratification based on individual risk profiles. Current biomarkers like CA125, HE4, p53, and emerging markers such as MSI, ARID1A, and miRNAs are transforming clinical practice by improving prognostic accuracy and supporting tailored therapeutic approaches.
As research continues to evolve, the integration of advanced technologies and collaborative efforts will drive the discovery of new biomarkers and refine existing ones. By overcoming challenges in standardization, validation, and ethical implementation, healthcare providers can harness the full potential of biomarkers to optimize patient care and outcomes in the management of endometrial cancer.
Implementing Biomarkers in Clinical Practice
Integrating prognostic biomarkers into clinical practice requires a systematic approach to ensure their effective utilization:
- Clinical Guidelines: Incorporating biomarker testing guidelines into standardized protocols and clinical pathways for consistent use across healthcare institutions.
- Education and Training: Providing ongoing education and training for healthcare providers on the interpretation and clinical implications of biomarker results.
- Patient Counseling: Educating patients about the role of biomarkers in their treatment journey, including informed consent for testing and understanding the implications of results.
- Multidisciplinary Collaboration: Facilitating multidisciplinary tumor boards or case conferences to discuss biomarker data and formulate personalized treatment plans.
- Quality Assurance: Implementing quality assurance measures to ensure accurate and reliable biomarker testing, including proficiency testing and external quality assessment programs.
Patient-Centered Care and Biomarkers
Incorporating biomarkers into patient-centered care involves:
- Shared Decision-Making: Engaging patients in discussions about biomarker testing and its implications for treatment decisions, aligning care with patient preferences and values.
- Monitoring and Follow-Up: Establishing protocols for monitoring biomarker levels during treatment and follow-up to assess treatment response, detect recurrence early, and adjust therapy as needed.
- Supportive Care: Providing psychosocial support and resources to help patients cope with the emotional and practical aspects of biomarker-informed treatment plans.
Regulatory and Ethical Considerations
Regulatory and ethical considerations in biomarker use include:
- Regulatory Approval: Ensuring biomarker tests meet regulatory standards for accuracy, reliability, and clinical validity before clinical implementation.
- Informed Consent: Obtaining informed consent from patients for biomarker testing, including disclosure of potential risks, benefits, and limitations.
- Data Privacy and Security: Safeguarding patient data and ensuring compliance with privacy regulations when storing and sharing biomarker information.
- Equitable Access: Addressing disparities in access to biomarker testing and ensuring equitable distribution of advanced diagnostic technologies across diverse patient populations.
Future Directions in Biomarker Integration
The future of biomarker integration in endometrial cancer care involves:
- Advancing Precision Medicine: Leveraging biomarkers to tailor targeted therapies and immunotherapies based on individual tumor profiles and molecular signatures.
- Personalized Risk Assessment: Developing predictive models that integrate biomarker data with clinical variables to refine risk stratification and optimize treatment algorithms.
- Health Technology Innovation: Harnessing artificial intelligence, machine learning, and digital health platforms to analyze big data sets and accelerate biomarker discovery and validation.
- Global Collaboration: Promoting international collaboration in biomarker research and clinical trials to enhance data sharing, validate findings across diverse populations, and expand access to innovative biomarker-based therapies.
Conclusion
Prognostic biomarkers are pivotal tools in the management of endometrial cancer, offering insights into tumor behavior, guiding treatment decisions, and improving patient outcomes. Current biomarkers like CA125, HE4, and emerging markers such as MSI and ARID1A are reshaping clinical practice by enhancing prognostic accuracy and facilitating personalized treatment strategies.
As biomarker research advances and technologies evolve, continued efforts in standardization, validation, and ethical implementation will be essential. By integrating biomarkers into comprehensive care pathways, healthcare providers can optimize treatment planning, monitor disease progression, and ultimately, improve the quality of life for patients with endometrial cancer.

Leave a Reply